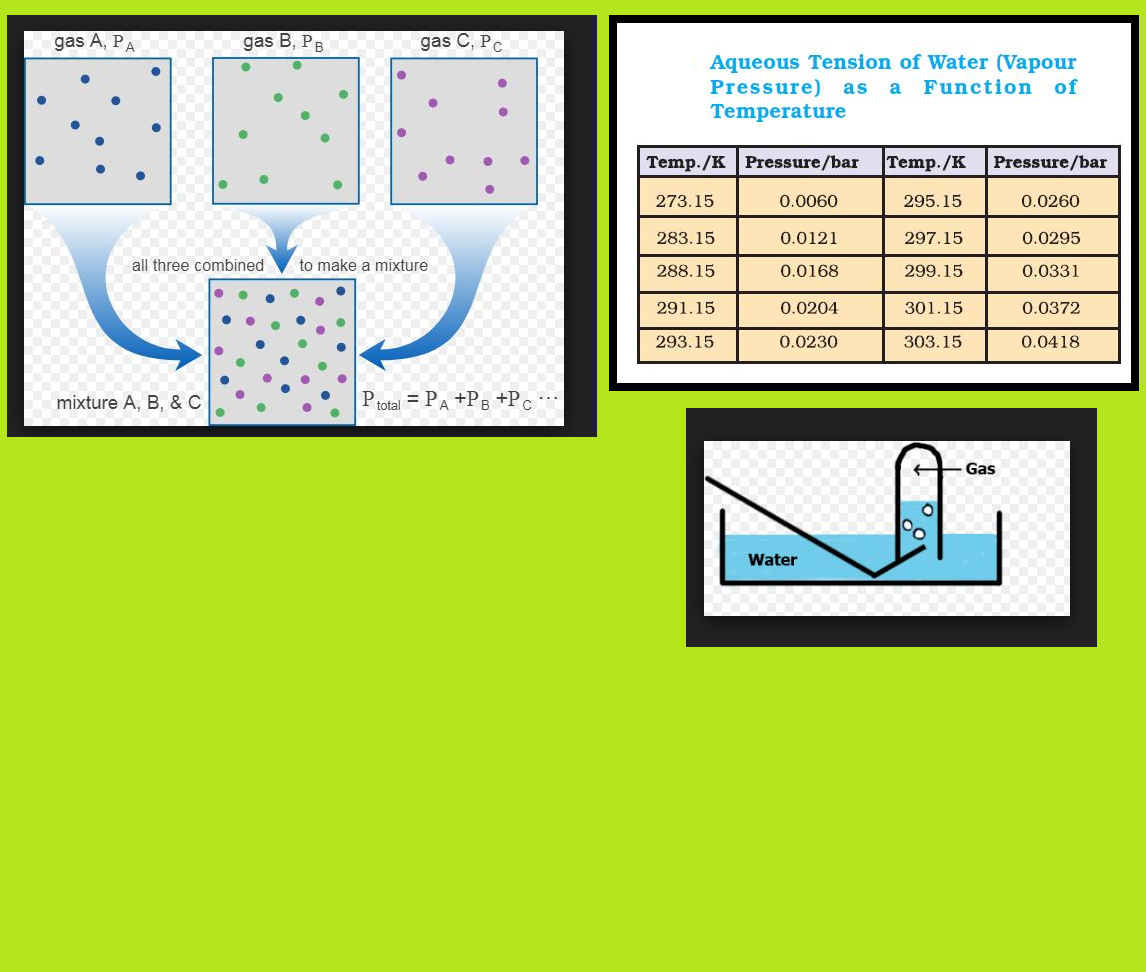
Ideal Gas Equation :
`text(Definition :)` The three laws which we have learnt till now can be combined together in a single equation which is known as ideal gas equation.
● At constant `T` and `n; V prop 1/p` Boyle’s Law
● At constant `p` and `n; V ∝ T` Charles’ Law
● At constant `p` and `T ; V ∝ n` Avogadro Law
● Thus `V prop (nT)/p` ...........(15)
`=> V = R (nT)/p` ...........(16)
where `R` is proportionality constant.
● On rearranging the equation (16) we obtain
`pV = n RT` .............(17)
`=> R = (p V)/(n T)` ..................(18)
● `R` is called gas constant.
● It is same for all gases.
● Therefore, it is also called `text(Universal Gas Constant)`.
● Equation (17) is called `text(ideal gas equation)`.
`=>` Equation (18) shows that the value of `R` depends upon units in which `p`, `V` and `T` are measured.
`=>` If three variables in this equation are known, fourth can be calculated.
● From this equation we can see that at constant temperature and pressure, `n` moles of any gas will have the same volume because
`V = ( n RT)/p`
and `n`, `R`, `T` and `p` are constant.
● This equation will be applicable to any gas, under those conditions when behaviour of the gas approaches ideal behaviour.
● Volume of one mole of an ideal gas under STP conditions (`273.15 K` and `1` bar pressure) is `22.710981 L mol^(-1)`.
● Value of `R` for one mole of an ideal gas can be calculated under these conditions as follows :
`R = ((10^5 pa) (22.71 ×10^(–3)m^3))/((1 mol) (273.15 K))`
`= 8.314 Pa m^3 K^(–1) mol^(–1)`
`= 8.314 × 10^(–2)` bar `L K^(–1) mol^(–1)`
`= 8.314 J K^(–1) mol^(–1)`
● At STP conditions used earlier (`0 °C` and `1` atm pressure), value of `R` is `8.20578 × 10^(–2) L atm K^(–1) mol^(–1).`
● Ideal gas equation is a relation between four variables and it describes the state of any gas, therefore, it is also called `text(equation of state)`.
● Ideal gas equation is the relationship for the simultaneous variation of the variables.
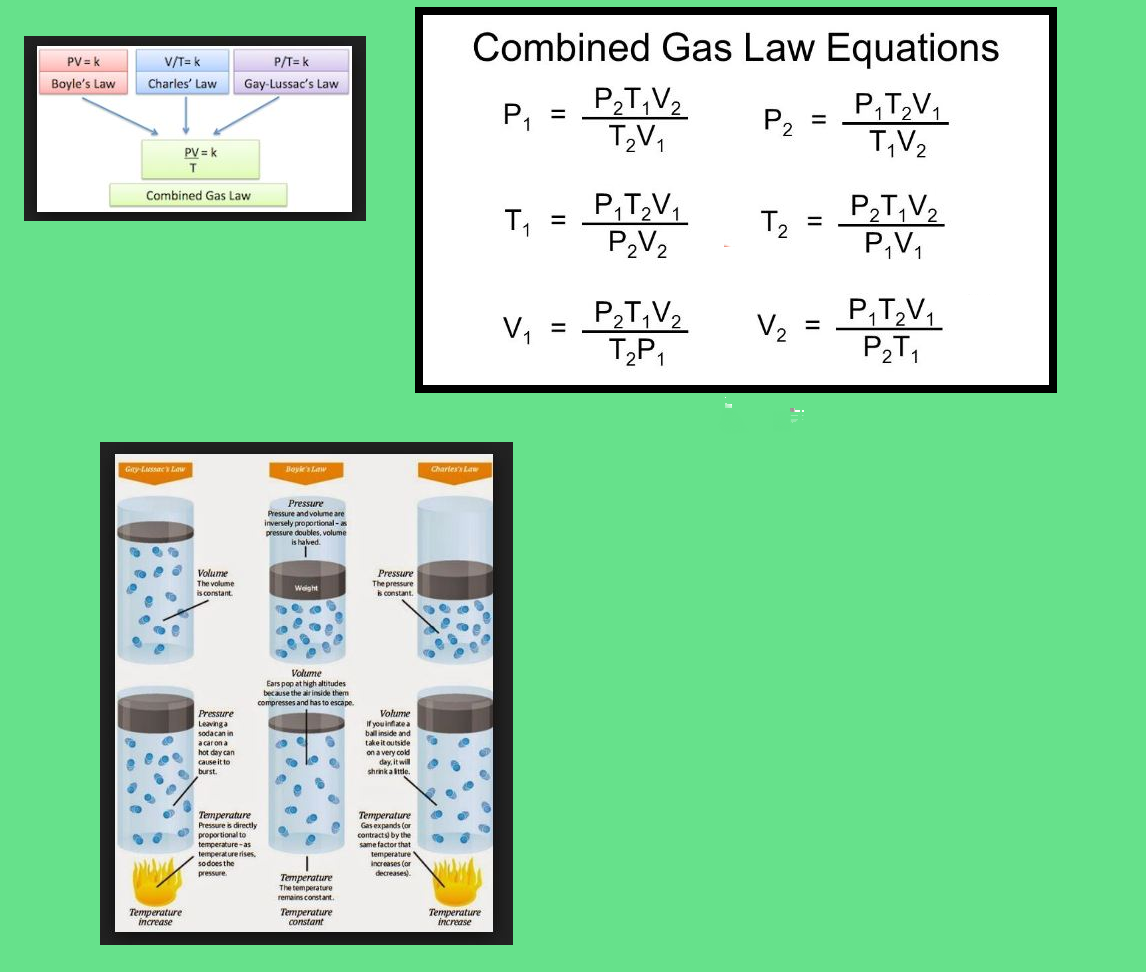
`=>` If temperature, volume and pressure of a fixed amount of gas vary from `T_1`, `V_1` and `p_1` to `T_2`, `V_2` and `p_2` then we can write
`(p_1 V_1)/T_1 = nR` and `(p_2 V_2)/T_2 = nR`
`=> (p_1V_1)/T_1 = (p_2 V_2)/T_2` .........(19)
● Equation (19) is a very useful equation.
● If out of six, values of five variables are known, the value of unknown variable can be calculated from the equation (19).
● This equation is also known as `text(Combined gas law)`.
● At constant `T` and `n; V prop 1/p` Boyle’s Law
● At constant `p` and `n; V ∝ T` Charles’ Law
● At constant `p` and `T ; V ∝ n` Avogadro Law
● Thus `V prop (nT)/p` ...........(15)
`=> V = R (nT)/p` ...........(16)
where `R` is proportionality constant.
● On rearranging the equation (16) we obtain
`pV = n RT` .............(17)
`=> R = (p V)/(n T)` ..................(18)
● `R` is called gas constant.
● It is same for all gases.
● Therefore, it is also called `text(Universal Gas Constant)`.
● Equation (17) is called `text(ideal gas equation)`.
`=>` Equation (18) shows that the value of `R` depends upon units in which `p`, `V` and `T` are measured.
`=>` If three variables in this equation are known, fourth can be calculated.
● From this equation we can see that at constant temperature and pressure, `n` moles of any gas will have the same volume because
`V = ( n RT)/p`
and `n`, `R`, `T` and `p` are constant.
● This equation will be applicable to any gas, under those conditions when behaviour of the gas approaches ideal behaviour.
● Volume of one mole of an ideal gas under STP conditions (`273.15 K` and `1` bar pressure) is `22.710981 L mol^(-1)`.
● Value of `R` for one mole of an ideal gas can be calculated under these conditions as follows :
`R = ((10^5 pa) (22.71 ×10^(–3)m^3))/((1 mol) (273.15 K))`
`= 8.314 Pa m^3 K^(–1) mol^(–1)`
`= 8.314 × 10^(–2)` bar `L K^(–1) mol^(–1)`
`= 8.314 J K^(–1) mol^(–1)`
● At STP conditions used earlier (`0 °C` and `1` atm pressure), value of `R` is `8.20578 × 10^(–2) L atm K^(–1) mol^(–1).`
● Ideal gas equation is a relation between four variables and it describes the state of any gas, therefore, it is also called `text(equation of state)`.
● Ideal gas equation is the relationship for the simultaneous variation of the variables.
`=>` If temperature, volume and pressure of a fixed amount of gas vary from `T_1`, `V_1` and `p_1` to `T_2`, `V_2` and `p_2` then we can write
`(p_1 V_1)/T_1 = nR` and `(p_2 V_2)/T_2 = nR`
`=> (p_1V_1)/T_1 = (p_2 V_2)/T_2` .........(19)
● Equation (19) is a very useful equation.
● If out of six, values of five variables are known, the value of unknown variable can be calculated from the equation (19).
● This equation is also known as `text(Combined gas law)`.